Data Acquisition: Comprehensively Covering Environmental Information
The Environmental Monitoring System (EMS) has a powerful data collection function. It can conduct real-time monitoring of analog quantities such as temperature, humidity, pressure difference, and gas concentration in the factory buildings. At the same time, it can display status quantities like door magnetic signals, ensuring the comprehensive acquisition of environmental information without missing any details. It is like a precise environmental information network, providing accurate data support for the pharmaceutical production environment.
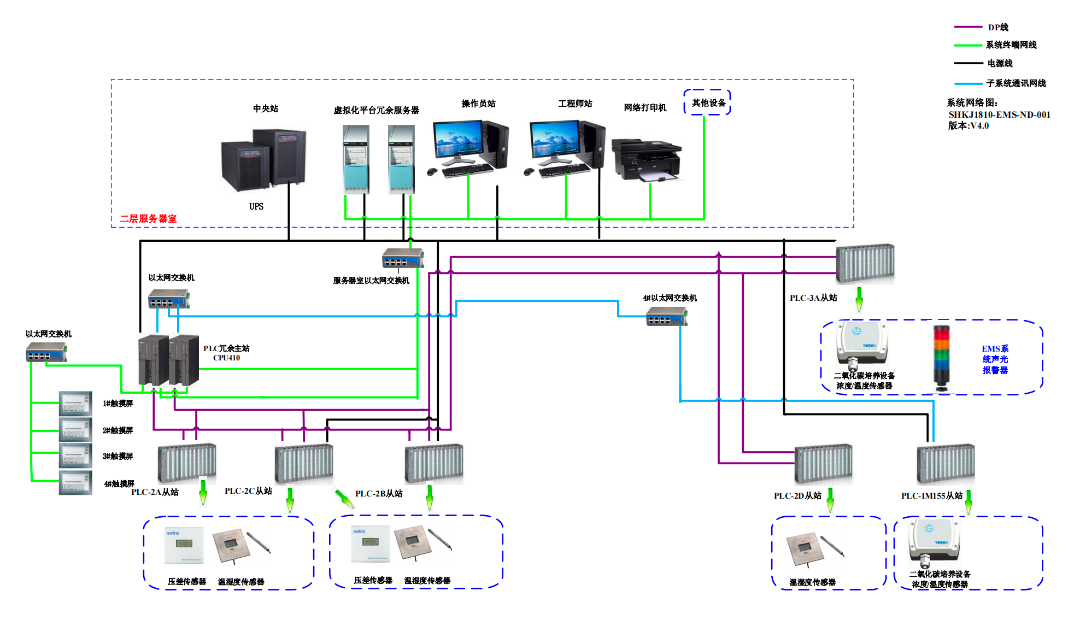
Data Processing: Smart Analysis for Efficient Decision-Making
In terms of data processing, the system can conduct various treatments on analog quantities. For example, alarm handling ensures the timely detection of abnormal situations, while data storage, recording, report printing, and archiving provide the basis for subsequent analysis. The processing of status quantities can convert the 0/1 status into meaningful information, such as the open/closed status of doors. Through a series of intelligent processing methods, enterprises can make efficient decisions and ensure the stability of the pharmaceutical production environment.
Alarming: Multi-Channel Early Warning
The alarm function is an important component of the Environmental Monitoring System (EMS). In addition to the text flashing and color changing on the host computer screen, as well as the audible and visual alarm of the alarm lamp, it also provides multi-dimensional alarm methods such as emails and text messages, which can directly send abnormal information to relevant responsible persons. Whether it is the abnormal temperature and humidity in the pharmaceutical workshop or the change in pressure difference, it can be notified in the first place, ensuring that product production is carried out in line with environmental standards and building a solid defense line for the safety of drugs.
Compliance Cornerstone: Comprehensive Control
Wellthinic Technology delivers GxP-compliant Installation Qualification (IQ) and Operational Qualification (OQ) protocols, adhering to regulatory-recognized software validation best practices and GAMP5 guidelines. It ensures that the system meets the strict standards of the pharmaceutical industry during the installation and operation processes, providing reliable quality assurance for pharmaceutical production enterprises. It offers a complete set of system validation services. From the meticulous planning in the project planning stage, to various evaluations in the requirement communication stage (including the Validation Plan VP, System GxP Impact Assessment, Regulatory Risk Analysis, User Requirements Specification URS/Functional Requirements Specification FRS, and Risk Assessment Report RA), then to the formulation of specifications in the system installation and configuration stage, as well as the Installation Qualification IQ, Operational Qualification OQ, Performance Qualification PQ, Requirements Traceability Matrix RTM, and Validation Summary Report VSR in the system validation stage, and finally to the acceptance report in the project delivery stage, every step is strictly controlled to ensure that the performance and compliance of the system reach the optimal level.

Wellthinic Technology boasts a professional team for automated installation and post-sales maintenance, delivering comprehensive support tailored to user needs. Following project evaluation, we customize cabinet configurations based on specific system requirements to ensure precise and compliant installation.

Address: Room 3134, 3rd Floor, No. 158 Liyi Road, Xiaoshan District, Hangzhou, Zhejiang Province 311215, P. R. China
Address: 3rd Floor, Building T6, No. 308 Kangshan Road, Pudong District, Shanghai, 201315, P.R.China

Address: 11th Floor, No. 18 Zhihui Road, Huishan District, Wuxi, Jiangsu Province 214174, P. R. China
