Interpreting Guidelines for Efficient Compliance
With the update of GAMP5, Wellthinic Technology attaches great importance to related studies. It has organized the validation team and quality team on multiple occasions to actively participate in academic discussions held by ISPE. We are well aware that only by closely following the pace of regulatory updates and continuously learning new validation theories, directions, and implementation methods can we provide high-quality and compliant CSV services to customers, meeting the data integrity compliance requirements of products.

International Authoritative Certification
The ISPE certificate is issued by the International Society for Pharmaceutical Engineering (ISPE). The association is committed to leading changes and innovations in global pharmaceutical technologies and processes. It aims to promote the internationalization process of the Chinese pharmaceutical industry and keep abreast of the latest development trends in global pharmaceutical engineering. Its authority is widely recognized within the pharmaceutical industry.
Regulatory Focus
Data integrity is of great significance in the pharmaceutical and life sciences fields. The focus of this training includes discussions on regulations such as 21 CFR Part 11, as well as methods to meet the international regulatory requirements for electronic records and signatures.
Data Management
The importance of data integrity throughout the system and data lifecycle, inspection methods, as well as the application of data governance frameworks, maturity models, mapping tools, and quality risk management.
System Operation
The integrity of the data lifecycle, different regulatory requirements, data governance, the compliant operation of GxP regulatory systems, and the impact of spreadsheets, mobile devices, and cloud computing on data integrity.
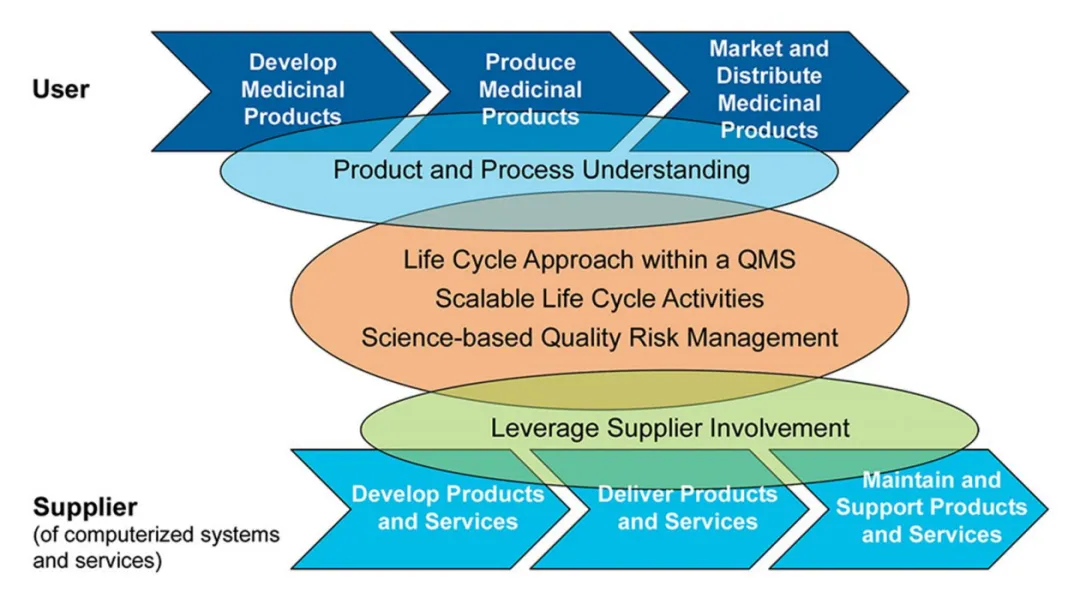
In the pharmaceutical industry and the field of life sciences, every production link is crucial for the safety and compliance of products. Therefore, enterprises have extremely high requirements for validation capabilities. Relying on decades of pharmaceutical validation experience and an international perspective, Wellthinic Technology provides customers with comprehensive CSV (Computerized System Validation) services, covering all aspects of computerized systems. From requirements analysis and design validation to installation qualification, operational qualification, and performance qualification, every step is carried out in strict accordance with regulatory requirements.
With the assistance of Wellthinic Technology, pharmaceutical enterprises can focus more on drug research and development and production, and provide safer and more effective products and services.

Address: Room 3134, 3rd Floor, No. 158 Liyi Road, Xiaoshan District, Hangzhou, Zhejiang Province 311215, P. R. China
Address: 3rd Floor, Building T6, No. 308 Kangshan Road, Pudong District, Shanghai, 201315, P.R.China

Address: 11th Floor, No. 18 Zhihui Road, Huishan District, Wuxi, Jiangsu Province 214174, P. R. China
