



Computerized System Validation (CSV) validation is an important part of quality assurance in the pharmaceutical and related industries, and it is a part that must be verified as stipulated in the GMP (Good Manufacturing Practice) appendices. The validation team of Wellthinic Technology has a profound background in the pharmaceutical industry and validation consulting. It has successively provided validation services for many well-known pharmaceutical companies, including foreign-funded enterprises and state-owned enterprises, as well as software companies. It is very familiar with computerized system validation, has the validation ability in line with GAMP5 guidelines, and has a profound understanding of and rich practical experience in both domestic and international GMP regulations.
It provides computerized compliance services in line with GMP, FDA and other regulations, meets the requirements of GMP computerized system verification, and reduces risks.
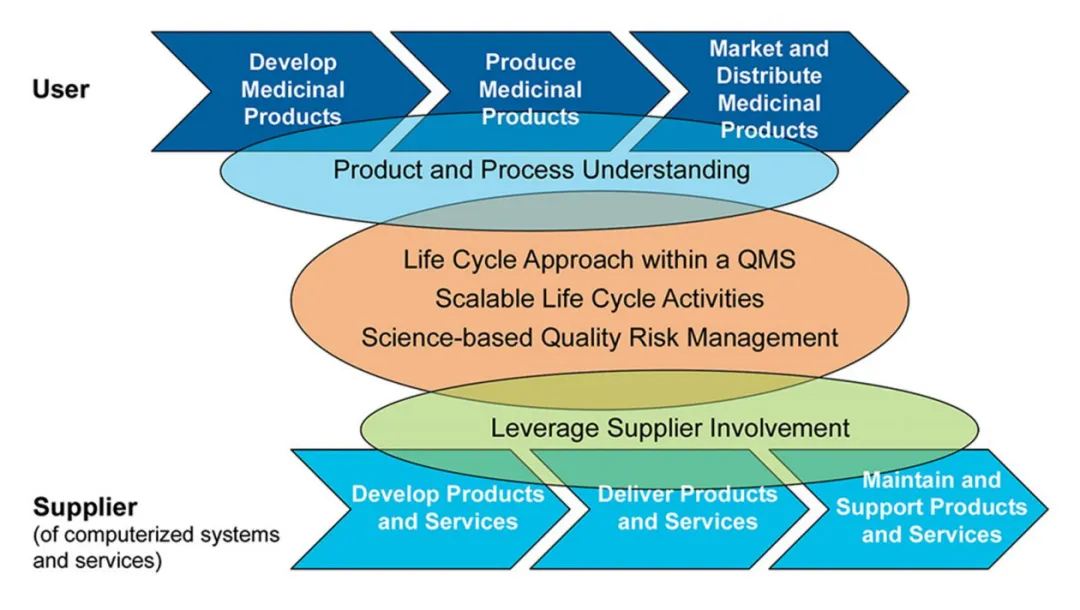
【Computerized system validation is provided based on the second edition of the GAMP5 guidelines to ensure that the validation plan keeps up with the times. Compliance reviews are carried out with reference to regulatory clauses to ensure data integrity.】
Advantages
• Implemented numerous projects for well-known domestic and international companies and provided a complete set of system validation documents.
• Conducted computerized system validation based on the second edition of the GAMP5 guidelines to ensure that the validation plan keeps pace with the times. Performed compliance reviews in accordance with regulatory clauses to guarantee data integrity.
• Replaced the linear model with an iterative CSV verification model. The validation process can be adjusted according to the complexity of the system, making the validation work more efficient.
• During the system commissioning and qualification process, more attention was paid to the completeness of function implementation and data compliance, by eliminating system risks through design is the optimal choice. Meanwhile, the system risk level can also be reduced through the commissioning and qualification process in response to the results of the system risk assessment.
• The integrated C&O method adopted the lifecycle verification flowchart from ASTM E2500 - "Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment" of the American Society for Testing and Materials and broke it down into specific implementable processes.
Highlights
• In accordance with regulations and combined with customers' needs, provide one-stop services of system consultation and CSV (Computerized System Validation) verification with extremely high cost-effectiveness.
• Strictly based on the results of the system risk assessment, comprehensively customize the CSV validation strategy and content.
• Have a mature project management system, a professional team of CSV engineers, and customized validation protocols.
All team members have a background in the pharmaceutical industry, are highly experienced, have a deep understanding of GxP regulations, and possess rich experience in CSV validation within the GxP field. They can accurately identify the risks of various systems. Ensure that the impact on the execution of customers' existing businesses is minimized and the project execution efficiency is maximized.

Address: Room 3134, 3rd Floor, No. 158 Liyi Road, Xiaoshan District, Hangzhou, Zhejiang Province 311215, P. R. China
Address: 3rd Floor, Building T6, No. 308 Kangshan Road, Pudong District, Shanghai, 201315, P.R.China

Address: 11th Floor, No. 18 Zhihui Road, Huishan District, Wuxi, Jiangsu Province 214174, P. R. China




